SpinalStim Spinal Fusion Therapy
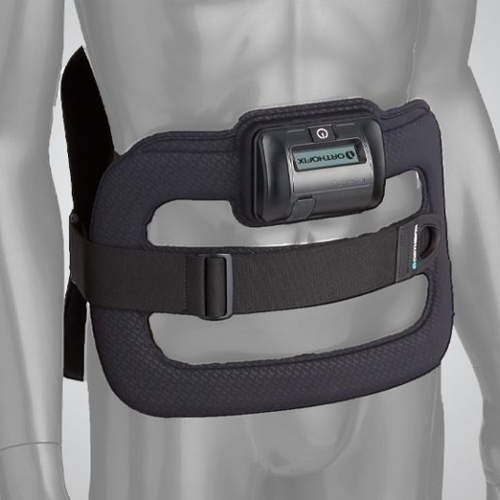
SpinalStim Spinal Fusion Therapy
The SpinalStim™ device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery.1-3 The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall clinical success rate of 92% in treating spinal fusion surgery patients.1-3 In addition, the SpinalStim device can be used for treatment of a failed spinal fusion, reducing the need for a revision surgery.
